Thank you to the researchers that submitted a poster for the 2024 BCI Battery Poster Research Showcase!

Investigation of organic expander molecules to advance understanding of structure-function relationships in lead acid batteries
Cailin Buchanan, Argonne National Laboratory, 2024 Winner
Expander molecules like Vanisperse A are added to the negative electrode pastes used in lead acid (PbA) batteries to promote high surface area and favorable discharge performance. Despite these advantages, expander molecules typically inhibit charging rates, limiting the use of PbAs in advanced applications that require repeated deep discharge/charge cycling. A deeper understanding of the atomic-level mechanisms that control additive-lead species interactions is necessary to optimize expander molecules for both discharge and charge performance. A collaborative project between government, academia, and industry has screened over one hundred model expander molecules (MEMs) using cyclic voltammetry, density functional theory, and various spectroscopy methods to characterize their chemical and electrochemical stability and performance properties. The MEMs are categorized by their lignin structural motifs and the presence of functional groups, e.g., sulfate, sulfonate, and carboxylate, with the goal of establishing structure-function relationships. Discharge (DEF) and charge (CEF) enhancement factors were established as the metrics for electrochemical performance relative to sulfuric acid without expanders as the baseline. Four categories of expander molecules were defined based on their DEF and CEF values: traditional, e.g., Van A, inhibitors, enhancers, and rheology modifiers. The set of materials evaluated to date demonstrates that expander molecules that can enhance both the discharge and charge performance are possible and do exist. On the other hand, the inhibitor class may lead to a deeper understanding of the expander degradation processes and their impact on cycle life. These results help us identify the design rules for expander molecules targeted to advanced PbA applications.

Design, synthesis and structural evaluation of model expander molecules for advanced lead-acid battery storage applications
Madhu Chennapuram, The University of Toledo, 2024 Winner
Lead acid batteries provide a day-to-day reliable energy storage application in various fields like automotive, standby power, renewable energy, telecommunication, industrial and robotics. 1 In addition to the electrochemically active lead species, these batteries contain a number of additives that improve performance and cycle life. Lignosulfonates (LS) are organic biopolymers that are used as additives in a variety of applications, including the production of lead acid batteries (Figure 1) 2. In lead acid batteries, LS serve as organic expanders to improve the performance of the battery’s storage capacity and extending its service life, as well as by acting as a wetting agents and improving the conductivity of the electrolyte, which tends to improve battery efficiency. 3 To be able to understand the interaction of specific functional groups with lead species in detail, small molecules that mimic portions of LS can be used as model expander molecules (MEMs). In this study, we are designing and synthesizing lignosulfonate-based MEMs. A series of MEMs were prepared from different synthetic methods (Scheme 1). The MEMs’ stability under conditions relevant for battery applications was investigated by cyclic voltammetry in 5 M H2SO4. Additionally, the interaction of the MEMs with Pb2+ and their stability at elevated temperature and in 5 M sulfuric acid was studied by spectroscopic and diffraction techniques.

Fundamental Studies of Lead Acid Batteries – Discharge Mechanism and Capacity Limits of Positive Active Materials
Frederick Agyapong-Fordjour, Argonne National Laboratory
The irreversible transition to clean renewable energy requires the deployment of energy storage systems at unprecedented scales. Lead batteries have the potential to continue serving as a crucial energy storage technology for a sustainable decarbonized energy economy because it is earth-abundant, inexpensive, and 99% recyclable. To advance the design of lead batteries with higher material utilization, fast recharge rates, and long-cycle life, will require a fundamental understanding of the electrochemical and chemical processes happening at the atomic and molecular scales. Here we discuss how the use of well-defined lead electrodes allows us to gain insights into the fundamental limits of discharge capacity and recharge rates. We unveil the relationships between discharge rates and PbSO4 particle size/layer thickness that ultimately governs the maximum discharge capacity accessible as a function of discharge rate on both the negative and positive lead electrodes. This data helped us develop a mathematical model that captures the key processes concerning lead ion and (bi)sulfate ion gradients coupled to nucleation and growth that explain, from first principles, the origin of the well-known empirical Peukert law. In this context, we explored how variables such as acid concentration, temperature, and the presence of lignosulfonate additives in the electrolyte further influence discharge capacity, allowing us to understand how thermodynamic, kinetic, and mass transport play a huge role in controlling the accessible capacity of the electrodes. This study paves the way for an in-depth understanding of the important variables in designing a lead acid battery performing at its full potential.

Non-stoichiometry and its influence in the Positive Active Material and Corrosion Layer of Lead Acid Batteries
Tiffany Kinnibrugh, Argonne National Laboratory
The malleable nature of lead oxides ranging from PbO to PbO2 plays a significant role in the positive electrode of lead batteries. Drawing from decades of thermal studies, engineering a conductive PbO1+x phase in the corrosion layer is necessary to avoid premature capacity loss driven by stoichiometric PbO. Within the active material, both α- and β-PbO2 are thought to be nonstoichiometric, with both oxygen [2] and Pb vacancies that are accompanied by proton interstitials[1]. The importance of non-stoichiometric retention was found in a neutron study which monitored the lead site’s vacancies in the PAM for conventional and fast charged batteries and showed a decrease from βPbO2 stoichiometry through the first 250 cycles with the fast charged battery continuing 750 cycles further than the conventional battery[3]. Our work sets out to provide a better understanding of non-stoichiometric phases and the conditions where they form to improve battery life and performance. We revisited PbOx phases associated within the corrosion layer using in situ diffraction and x-ray absorption and found the presence of metastable Pb3O5 and Pb2O3 phases during thermal decomposition of β-PbO2. Separately, changes in the stoichiometry of β-PbO2 in an electrochemical environment were also investigated using in situ diffraction during cycling of a Planté cell at oxidizing conditions. Both studies were supported by density functional theory calculations which provide context on the overall stability and structure of these phases and the origins of their electronic and oxygen mobility.

Advancing Lead Acid Batteries: Comprehensive Insights into the Evolution of Active Materials and Interfacial Processes through Multimodal Analysis
Vijayakumar Murugesan, Pacific Northwest National Laboratory
The lead-acid battery is a time-tested and robust rechargeable battery technology that has withstood the test of time and is still widely applicable owing to its reliability and high power-to-weight ratio. However, its integration into grid-scale stationary energy storage applications faces challenges, as its performance lags newer battery chemistries. The charge-discharge cycles in lead-acid batteries involve multiscale chemical, morphological, structural, and microstructural evolution, driven by repeated dissolution-nucleation-precipitation of active materials under an applied electric field. Understanding these interfacial-driven processes at the atomistic and molecular scales, as well as elucidating the role of additives facilitating these multiscale transformations, are crucial for enhancing battery life and performance. At PNNL, we have adopted a multimodal analysis approach to uncover the underlying mechanisms occurring at the electrode-electrolyte interfaces in Pb-acid batteries. By utilizing a combination of in-situ and ex-situ tools, we address the most challenging issues surrounding the dissolution-nucleation and phase transformation regimes of active materials. This presentation will discuss our efforts to provide atomistic insights into PbOx phase evolution during in-situ heating, PbSO4 nucleation over native and strontium-doped barite surfaces, and the ion dynamics of sulfuric acid-based electrolytes, all derived from our multimodal analysis. Our goal with these multimodal analyses is to establish a predictive regime for designing active materials with outcomes that can withstand the harsh demands of grid-scale stationary energy storage applications.

Evaluation of Model Expander Molecules in Lead Batteries
Sahar Nazeer, University of Toledo
Lead acid batteries represent the oldest rechargeable battery technology, still widely used. In addition to automotive applications, exploring their application for large-scale energy storage is desirable. However, the sulfation process in the battery is affecting its charging capacity and performance. Lignosulfonates, which are also referred to as “negative expanders”, are used as an additive in lead acid batteries to overcome some of the issues related to sulfation. Negative expanders perform versatile functions in batteries. They affect the morphology of lead sulfate crystals, the rate of oxidation of lead sulfate, and reduce the sintering of the porous active mass. Lignosulfonates are complex molecules with multiple functional groups, but which functional group interacts with the battery component and improves the performance is still unknown. So, model expander molecules that resemble the various groups of these commercial expanders were synthesized instead. The main motivation of this project was to analyze these interactions and how these interactions can be exploited to enhance the performance of lead batteries. In this research, the synthesis of various MEMs was accomplished by alkylation and sulfonation reactions. The purity of the MEMs was characterized by proton nuclear magnetic resonance (H-NMR) spectroscopy and elemental analysis. Moreover, electrochemical properties were investigated by Cyclic Voltammetry. Their effect on the charge/discharge behavior of lead electrodes in 5 M sulfuric acid was studied by collaborators at Argonne National Laboratory, and several compounds were found to improve charge, discharge, or both.

Novel Characterization of Pb-acid Materials Utilizing XRM paired with Micro-CT
Grant Spencer, University of North Texas, Department of Materials Science and Engineering
Lead-acid (Pb-acid) battery cells are a growing electrochemical choice for renewable energy grid-storage, while remaining the premier choice for combustion engine start-up sources. Up until recently, very few non-destructive experimentation techniques have been utilized for Pb-acid cell characterization – such techniques are useful to identify performance-defining heterogeneities that may develop within a cell during electrochemical cycling. X-ray microscopy (XRM) is a non-destructive, advanced characterization technique that, when paired with micro-computational tomography (µCT), renders 3-dimensional images that can be utilized to analyze features with resolution dimensions as small as 700 nm, depending on sample material, thickness, and geometry. This study applied XRM with µCT to analyze Pb-acid minicells and such performance-defining heterogeneities that might develop under voltametric cycling, such as secondary phase growth and evolution, and crack and porosity formation, as well as inclusions. Utilizing ORS Dragonfly software for 3D image refinement and final stage analysis, large phase transformation was recognized for the Pb-paste within the cell between initial and final volumes, when considering secondary phase growth. Feature sizes with resolutions of 4-5 microns were observed using the NDE technique. Due to XRM’s large depth of field, this technique is considered advantageous for region of interest analysis for more in-depth studies involving Pb-based materials, that include smaller feature sizes, below submicron range, as well as for a selection stage for region of interest investigation utilizing scanning electron microscopy (SEM) or transmission electron microscopy (TEM), which was utilized for investigation of features with nm sizes.

Effect of Model Expander Molecules on the Morphology of Lead Sulfate Crystals
Radha Shah, University of Toledo
Rechargeable lead-acid batteries have found widespread use over the past century, with applications ranging from the commonly encountered SLI (starting, lighting, ignition) batteries in motor vehicles to applications like backup power storage for critical infrastructure. The global drive to reduce dependence on fossil fuels and increase the use of renewable energies requires the development of vast amounts of reliable and safe energy storage. Lead batteries are attractive for such applications because of the high abundance of raw materials, excellent recycling of spent batteries, reliability and safety. However, current batteries suffer from limitations during deep discharge or rapid charging, which must be overcome if they are to be used for grid storage. During the discharge of lead-acid batteries, lead sulfate forms on both the negative and positive electrodes of the battery; this is a process known as sulfation. The morphology of the lead sulfate particles has a strong effect on the reversibility of this process when the battery is recharged. It is known that problems for battery life arise when coarse, needle-like lead sulfate deposits form on the electrodes of the batteries. Such deposits contribute to gradual inhibition of the battery. The electrodes in lead-acid batteries contain additives to the electroactive lead species that improve the cycling performance of the batteries. These include carbon, barium sulfate and lignosulfonates (LS), which are referred to as expanders. The presence of these compounds affects the morphology of the lead sulfate particles formed on the electrodes, and through this the ease of reversing the initial discharge reaction. LS are known to enhance discharge rates several fold, but interfere with rapid charging. However, it is not known what functional groups on the lignosulfonates play specific roles in this process. Smaller molecules that mimic specific functional groups on lignosulfonates, which are referred to as “model expander molecules” (MEMs), can be used to explore such effects in more detail. In this poster, the effect of MEMs on the morphology of PbSO4 was explored by carrying out precipitation reactions by controlled addition of Pb2+ and sulfate solutions. Scanning electron microscopy was used to evaluate differences in morphology, while phase identification and evaluation of crystallinity was carried out by power x-ray diffraction.

Nucleation and Growth of Barium Sulfate as a Proxy for Lead Sulfate in Lead-Acid Batteries
Andrew Stack, Oak Ridge National Laboratory
Lead acid battery charge and discharge rates and efficiency likely depend in part on the texture of lead sulfate crystals. That is, the distribution, size and plate-connectivity of the crystals during their formation in the discharge cycle likely influence their dissolution rates during the charging cycle. Here, we use the precipitation of a natural material, barium sulfate, as an analog to establish a fundamental basis to understand how lead sulfate texture might be affected by the rate of nucleation and growth, as well as additives to solution, such as electrolyte ions. The work may have provide some guidance on a strategy to tailor lead-acid battery composition and cycling characteristics for specific applications.
Past Innovation Award Submissions
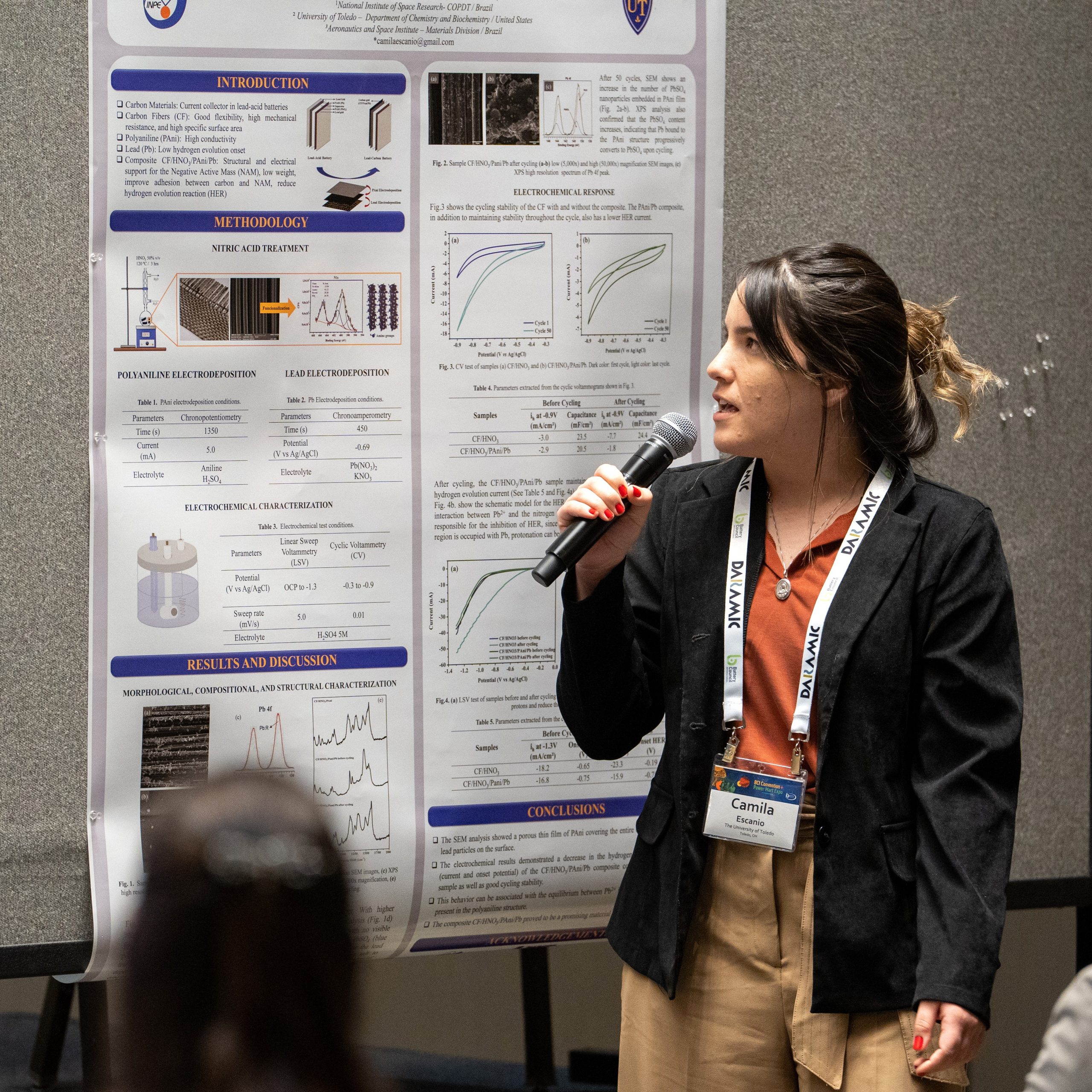
Carbon Fiber/Polyaniline/Lead Composite With Low Hydrogen Evolution Activity With Potential Application in Lead Acid Battery
Camila Alves Escanio, The University of Toledo/National Institute for Space Research
A composite made of Carbon fiber (CF), Polyaniline (PAni), and lead (Pb), with low hydrogen evolution activity, was developed and studied as a potential material to be used in lead acid batteries. First, the CF was subjected to acid treatment using 50% v/v HNO3 to remove the sizing and add nitrogen functional groups on the carbon surface (CF/HNO3). The second step, PAni electrodeposition (CF/HNO3/PAni), was performed through chronopotentiometry by applying a constant current of 5 mA for 1350 s. Next, the lead electrodeposition was carried out by chronoamperometry by applying the lead reduction potential (-0.69 V vs Ag/AgCl) for 450 s. Finally, the composite CF/HNO3/PAni/Pb was dried in a vacuum oven at 60 °C for 1 h. Morphological and structural analyses were performed using Scanning Electron Microscopy (SEM) and Raman Spectroscopy. In addition, the electrochemical behavior, such as the hydrogen evolution phenomenon and cycling stability, was evaluated by Linear Sweep Voltammetry and Cyclic Voltammetry, respectively. The SEM analysis showed a porous thin film of PAni covering the entire carbon fiber with some lead particles on the surface. Furthermore, the electrochemical results demonstrated a decrease in the hydrogen evolution phenomenon (current and onset potential) of the CF/HNO3/PAni/Pb composite compared to the CF/HNO3 sample as well as good cycling stability. Acknowledgment: The authors are grateful to FAPESP (process nº 2022/10542-4 and nº 2019/08473-1) for the financial support.

Fundamental Studies of Lead Batteries – Trace Level Surface Composition Dynamics
Pietro Papa Lopes, Argonne National Laboratory
Lead batteries are only possible because of surface kinetic limitations that exist when evolving H2 over high-purity pure Pb and O2 over PbO2 electrode materials. These “gassing” reactions are secondary processes that may occur during overcharge and are highly influenced by the presence of transition metal impurities in the electrolyte, or due to the presence of Sb atoms arising from the corrosion of positive electrode plates. However, common elements present in part per million levels in secondary lead sources such as Bi, Ag, and Tl have been far explored in how they contribute to these secondary processes, particularly for the H2 evolution reaction (H2 gassing) at the negative electrode surfaces. In this study, we show how the presence of Bi, Ag, and Tl as dopants in Pb surfaces at concentrations ranging from tens to hundreds of ppm influence H2 gassing rates. Furthermore, by employing a new electrochemical in situ method that uses a stationary probe disk electrode setup (SPRDE) connected to an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) instrument we were able to measure in real-time the surface dissolution processes for each dopant of interest as a function of the electrode potential. These experiments revealed selective surface dissolution from Tl atoms in the surfaces before lead discharge occurs, as well as consequent removal of traditionally more electrochemical stable elements such as Bi during lead dissolution as the first step in the discharge mechanism. We show how the removal of these trace-level impurities decreases the H2 gassing rates, but when present in the electrolyte at sufficient concentrations, the trend is reversed and H2 gassing may accelerate due to surface redeposition. Our results offer new insights into the existence of surface composition dynamics that play an important role in the secondary processes and may offer new guidelines in the design of ultra-low gassing batteries.
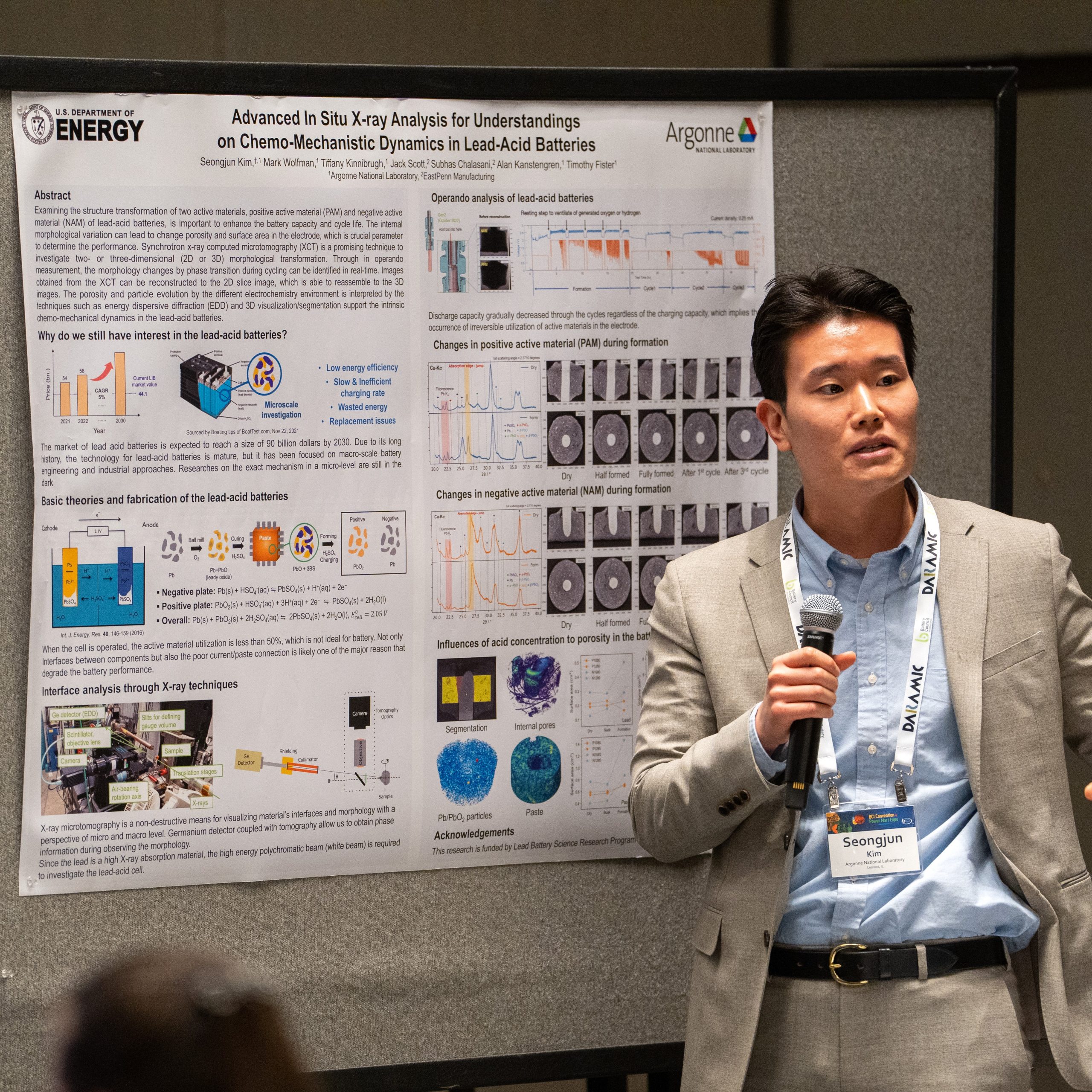
Advanced In-Situ X-Ray Analysis for Understandings on Chemo-Mechanistic Dynamics in Lead-Acid Batteries
Seongjun Kim, Argonne National Laboratory
Since developed in 1859, the lead-acid batteries have been loved as secondary power source for various vehicles because of its cheap price and easy production process. Although the lead-acid battery is inferior in terms of specific energy and power density from the high atomic weight Pb compared to state-of-art lithium-ion batteries (LIB), this classical system has still loved worldwide all times because of its bold economic advantages. High powder density and simplicity of manufacture solidifies their status in the battery market. In addition, it is noteworthy that the almost every component after use can be recycled and long storage time in the perspective of energy sustainability. However, the lead-acid battery should overcome some major problems in order to compete other types of batteries. Deep discharge cause facilitation of rapid sulfation, also the irreversible crystallization of lead sulfate (PbSO4) can decrease the battery lifespan and performance. In other words, examining the structure transformation of two active materials, positive active material (PAM) and negative active material (NAM), is important to enhance the battery capacity and cycle life. The internal morphological variation can lead to change porosity and surface area in the electrode, which is crucial parameter to determine the performance. However, the existing post-mortem analysis such as scanning, and transmission electron microscopy (SEM and TEM) do not show the real-time changes during cycling. Synchrotron x-ray computed tomography (XCT) is a promising technique to investigate two- or three-dimensional (2D or 3D) morphological transformation. Through in situ or in operando measurement, the morphology changes by phase transition during cycling can be identified in real-time. Images obtained from the XCT can be reconstructed to the 2D slice image, which is able to reassemble to the 3D images. The porosity and particle evolution by the different state of charge (SOC) is interpreted by the techniques. Additionally, the other synchrotron x-ray techniques such as diffraction (XRD) and transmission x-ray microscopy (TXM) support the intrinsic chemo-mechanical dynamics in the lead-acid batteries. This presentation will provide much interest in the lead-acid batteries and information on the mechanism analysis using various synchrotron x-ray techniques. The application of in situ and operando x-ray measurements enable to enhance general comprehensions on the battery chemistry beyond not only lead-acid but other types of batteries.

Synthesis of Model Expander Molecules To Investigate the Effect of Lignosulfonate Additives in Lead Batteries
Brandon Russell, The University of Toledo
Despite their long use, lead acid batteries are not fully understood. While the electrochemical properties of Pb/PbSO4 oxidation and reduction are known, the exact effects and stability of additives have not been optimized. Lignosulfonates, which are added as expanders to the negative plate, have been shown to improve discharge behavior while limiting the chargeability of lead acid batteries. However, the atomic level interactions that result in these effects are unknown. Due to the complexity of lignosulfonate structure, the functional groups responsible for the enhancements and limitations observed cannot easily be determined. It has been hypothesized that the sulfonate groups present have a significant impact on battery performance. To test the impact of sulfonates and other functional groups, simpler molecules with specific functional groups are synthesized in this work to isolate the effect of each group. The effects of these model expander molecules, or MEMs, on performance and stability can then be compared to commercially used expanders or setups without additives. MEMs were sulfonated using either sodium sulfite or sulfuric acid depending on the targeted sulfonation site. NMR and IR spectroscopy were used to verify MEM modification and purity. EDS was used to confirm the presence of sulfur and absence of inorganic byproducts. IR spectroscopy and powder diffraction were used to detect interactions with lead ions, while the stability of MEMs in 5 M sulfuric acid was tested by cyclic voltammetry. Several MEMs that were successfully synthesized showed stability in 5 M sulfuric acid as well as the ability to coordinate with lead ions.

Synthesis and Purification of Model Expander Molecules for Advanced Lead-Acid Battery Storage Applications
Madhu Chennapuram, Department of Chemistry and Biochemistry, The University of Toledo
Lead acid batteries provide a day-to-day reliable energy storage application in various fields like automotive, standby power, renewable energy, telecommunication, industrial and robotics.1 In addition to the electrochemically active lead species, these batteries contain a number of additives that improve performance and cycle life. Lignosulfonates (LS) are organic biopolymers that are used as additives in a variety application, including the production of lead acid batteries. In lead acid batteries, LS serve as organic expanders to improve the performance of the battery’s storage capacity and extending its service life, as well as by acting as a wetting agent and improving the conductivity of the electrolyte, which tends to improve battery efficiency.2 LS are molecules with high molecular weights that possess a large number of different functional groups in their structure (Figure 1). Due to the complex structural feautures of LS, it is very hard to predict the active site of functional groups interact with lead species and affect battery performance. To be able to understand the interaction of specific functional groups with lead species in detail, small molecules that mimic portions of LS can be used as model expander molecules (MEMs) (Scheme 1). In this study, we are synthesizing lignosulfonate-based MEMs. A series of MEMs were prepared from commercially available alcohols via bromination and sulfonation. The MEMs’ stability under applied potential in 5 M H2SO4 was confirmed by cyclic voltammetry. References: 1) a) G. J. May, A. Davidson and B. Monhov. Journal of Energy storage. 2018, 15, 145-157. b) R. Katahira, T. J. Elder and G. T. Beckham. Lignin Valorization: Emerging Approaches, 2018, ISBN: 978-1-78262-554-4. 2) B. O. Myrvold. Journal of Applied Electrochemistry. 2005, 35, 573-579.

Morphology of Lead Sulfate Obtained in the Presence and Absence of Model Expander Molecules
Margaret Hannah, Department of Chemistry and Biochemistry, The University of Toledo
The lead-acid battery is a rechargeable battery that has been in frequent use for the past century, with applications ranging from the commonly encountered SLI (starting, lighting, ignition) batteries in a motor vehicle to the less common but emerging use of lead-acid batteries for large-scale energy storage. During the discharge of lead-acid batteries, lead sulfate forms on both the negative and positive electrodes of the battery; this is a process known as sulfation. When the lead sulfate that builds up consists of individual particles that are fine and uniform in nature, this process is reversible when the battery is recharged. Problems for battery life arise when coarse, needle-like lead sulfate deposits form on the electrodes of the batteries. Such deposits contribute to gradual inhibition of the battery. The electrodes in lead-acid batteries contain additives to the electroactive lead species that improve the cycling performance of the batteries. These include carbons, barium sulfate and lignosulfonates, which are referred to as expanders. The presence of these compounds affects the morphology of the lead sulfate particles formed on the electrodes, and through this the ease of reversing the initial discharge reaction. However, it is not known what functional group on the lignosulfonates may play a role in this process. Smaller molecules that mimic specific functional groups on lignosulfonates, named “model expander molecules” (MEMs) can be used to explore the effects in detail. In this poster, the precipitation of lead sulfate using controlled addition of Pb2+ to sulfate solutions in the absence and presence of these MEMs was explored. Scanning electron microscopy was used to evaluate differences in morphology of lead sulfate precipitated under a variety of different conditions. Phase identification and evaluation of crystallinity was carried out by power x-ray diffraction.

Brunauer-Emmett-Teller (Bet) Analysis for the Surface Area of Ingredients in Lead Acid Battery
Ravi Shankar Raju Madiraju, Hammond Group INC
As particles get smaller, their surface area to volume ratio rises. The multi-molecular adsorption technique “BET”, to determine the surface area was invented by Stephen Brunauer, Paul Hugh Emmett, and Edward Teller in 1938. Brunauer, Emmett, and Teller expanded Langmuir’s (1918) adsorption to several molecular layers to understand how adsorption data affects material attributes such as total surface area, pore-size distribution, micro-pore analysis, and porosity. BET method is used for both macro and nano materials and is a common technique used to classify raw materials and active materials in the battery industry. This study mainly concentrates on finding the correlation coefficient for each ingredient of a lead-acid battery paste mix against BET surface area values. This is done by developing a master data matrix that collects weight percentages of the ingredients in a lead acid battery paste mix with respect to lead-oxide content. The BET surface area results of the dry cured battery active material are then compared to the weight percentages of each ingredient to determine that ingredient’s effect on surface area. Correlation coefficients measure linear relationships between variables. -1 to 1 are its values. According to a correlation value of -1, one series rises while the other falls, and vice versa. 1 is a perfect positive connection. Zero correlation means no linear relationship.

Fundamental Studies of Lead Batteries – Insights on Discharge Capacity Limits From Well-Defined Negative Electrode Interfaces
Frederick Agyapong-Fordjour, Argonne National Laboratory
Lead batteries have the economic potential to continue serving as an important energy storage technology for a decarbonized sustainable energy future. Advancing the design of lead batteries with higher material utilization, fast recharge rates, and long-cycle life requires a fundamental understanding of the electrochemical and chemical processes taking place at the atomic and molecular scales. In this work, we will discuss how the use of well-defined lead negative electrode interfaces allows us to gain insights into the fundamental limits of discharge capacity and recharge rates. By starting from ultra-high purity, clean, and electropolished smooth lead surface with nanometer-scale surface roughness we were able to unveil the relationships between discharge rates and PbSO4 particle size/layer thickness that ultimately governs the maximum discharge capacity accessible as a function of discharge rate. This data helped us develop a mathematical model that captures the key processes concerning lead ion and (bi)sulfate ion gradients coupled to nucleation and growth that explain, from first principles, the origin of the well-known empirical Peukert law. In this context, exploring how variables such as acid concentration, temperature, and the presence of lignosulfonate additives in the electrolyte further influence discharge capacity allow us to understand how thermodynamic and kinetic parameters play a huge role in governing the accessible capacity from negative electrodes. This study paves the way to a deeper understanding of the variables that are most important in improving the discharge capacity of negative electrodes, such as the design of expander molecules.

Fundamental Studies of Lead Batteries – Lead Sulfate Particle Size Effect on Recharge Rates
Crystal Ferels, Argonne National Laboratory
Understanding the factors governing the recharge process of negative electrodes in lead batteries may open new opportunities in the design of advanced batteries for automotive applications as well as for grid energy storage systems. The conversion of lead sulfate back to metallic lead is a complex process that may involve chemical dissolution followed by electrochemical reduction of sparingly soluble lead ions at the negative electrode surface. Depending on the discharge conditions, rest periods, and overall battery operational protocol, lead sulfate crystals will vary in size and thus influence recharge rates. Here, we employed a well-defined electrochemical interface approach to gain insights into the recharge mechanism and understand the fundamental kinetic limits of the charging process. By using thickness-controlled lead sulfate layers prepared over lead surfaces and charging the electrode using a potentiostatic scan we were able to reveal how the kinetics of the chemical dissolution step from PbSO4 to Pb2+ ions is the rate-determining step of this reaction. In turn, this allows us to define a new metric called kinetic charge acceptance (KCA) that establishes how fast one can recharge a lead sulfate layer of a given layer thickness or equivalent particle size. Furthermore, we provide direct experimental evidence of how lead sulfate particle size distribution governs the recharge rates by using chemically synthesized PbSO4 particles with sizes ranging from 50 nm up to 1 micrometer deposited on carbon electrodes. The use of well-defined particles allowed us to observe an inverse relationship between particle size and recharge rates, namely, an increase in lead sulfate average particle size from 100 nm to 1000 nm decreases the measured KCA by a factor of 10. These results indicate the importance that controlling the lead sulfate particle size during discharge can have in the overall negative electrode rechargeability, and it also opens new opportunities to improve the formation of batteries during manufacturing.
I was fascinated by the many facets of lead batteries that I never knew existed…