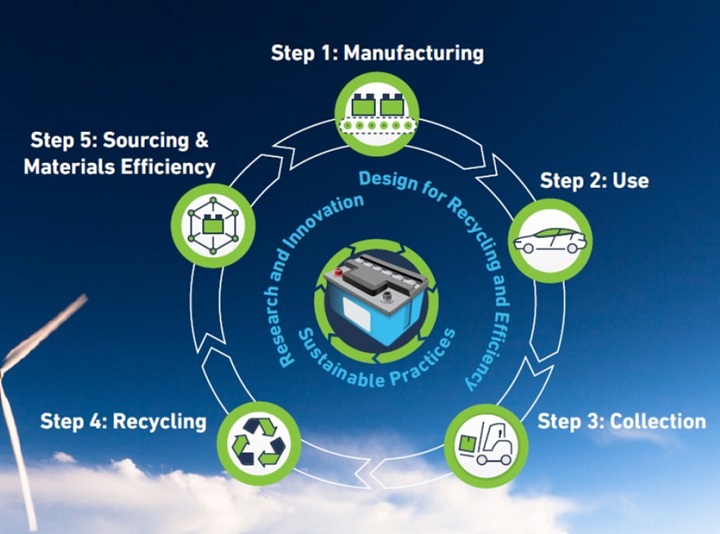
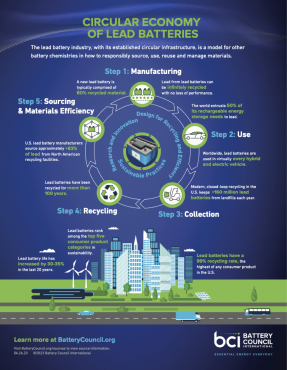
The U.S. lead battery industry’s commitment to safe and sustainable recycling methods ensures that lead batteries are an essential part of an energy storage mix to achieve a cleaner, greener future.
Lead Battery Industry Embodies...
As a pioneer in sustainability, the lead battery industry's near perfect rate of spent battery recycling is a true recycling success story...
Article
November 14, 2022